To study the quantity of Casein in different samples of milk Chemistry Investigatory Project PDF F Class 12
PDF Download Link Given Below
To Get the Link Scroll Down to end of this Page
INTRODUCTION
What is Casein?
Casein is the protein found in all mammals’ milk. Mammals include cow, goat, sheep, yak, buffalo, camel and humans. Milk is a complete diet as it contains minerals, vitamins, proteins, Carbohydrates, Fats and Water.
Average composition of milk from different sources is given below:
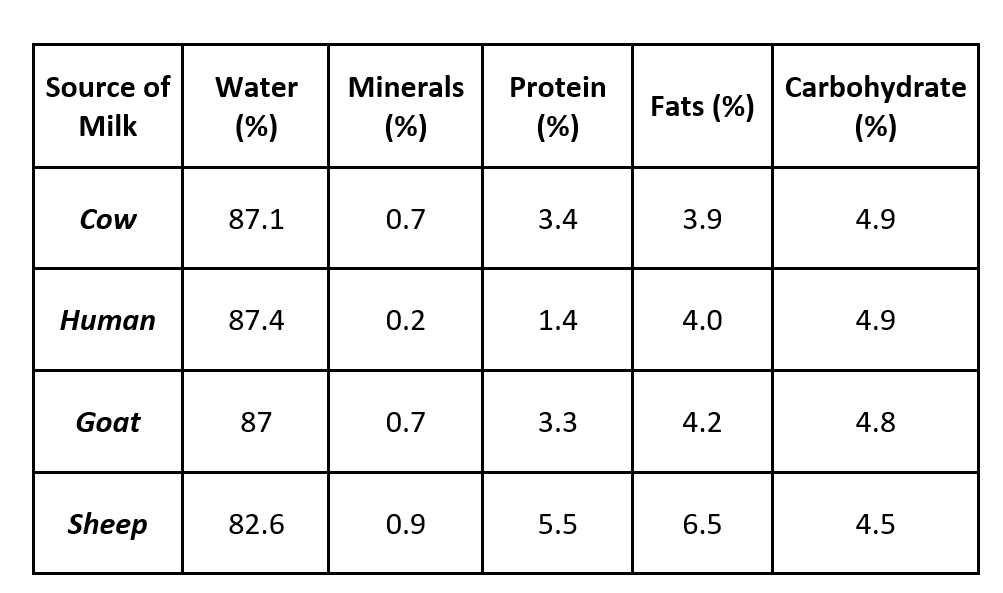
Casein in the most predominant protein in milk and is a mixed phosphor protein. Casein has an isoelectric pH of about 4.7 can be easily separated around this isoelectric pH. It readily dissolves in dilute acids and alkalies.
Casein is present in milk as calcium caseinate in the form of micelles. These micelles have negative charge and on adding acid to milk the negative charges are neutralized.
Natural milk is an opaque white fluid Secreted by the mammary glands of Female mammal the main constituents of natural milk are Protein, Carbohydrate, Mineral Vitamins, Fats and Water and is a complete balanced diet Fresh milk is sweetish in taste However, when it is kept for long time at a temperature of 5 degree it become sour because of bacteria present in air. These bacteria convert lactose of milk into lactic acid which is sour in taste. In acidic conditions casein of milk separate out as precipitate. When the acidity in milk is sufficient and temperature is around 36 degree, it forms curd.
REQUIREMENT
- Beakers (250 ml)
- Filter-paper
- Glass rod
- Weight box
- Filtration flask
- Buchner funnel
- Test tubes
- Porcelain dish
- Different samples of milk
- 1 % acetic acid solution
- Ammonium sulphate solution
THEORY
Natural milk is an opaque white fluid Secreted by the mammary glands of Female mammal. The main constituents of natural milk are Protein, Carbohydrate, Mineral Vitamins, Fats and Water and is a complete balanced diet. Fresh milk is sweetish in taste.
However, when it is kept for long time at a temperature of 5 degree it become sour because of bacteria present in air. These bacteria convert lactose of milk into lactic acid which is sour in taste. In acidic condition casein of milk starts separating out as a precipitate. When the acidity in milk is sufficient and temperature is around 36 degree, it forms semi-solid mass, called curd.
PROCEDURE
- Wash the beaker (250 ml) with the distilled water and dry it.
- Take 20 ml of buffalo’s milk in 250 ml beaker and find its weight.
- Add 20 ml saturated solution of ammonium sulphate slowly with stirring. Fat and casein will separate out as precipitate.
- Filter the above solution and transfer the precipitate in another beaker.
- Treat the above precipitate with 30 ml distilled water. Casein dissolves forming milky solution whereas fat remains as such.
- Warm the above contents of the beaker to 40 – 45°C on a low flame. Now, add 1% acetic acid solution drop wise with stirring when casein gets precipitated.
- Filter the precipitated casein and wash with distilled water and dry it.
- Find the weight of dry precipitate.
- Repeat the whole experiment with cow’s milk, goat’s milk and sheep’s milk.
OBSERVATION
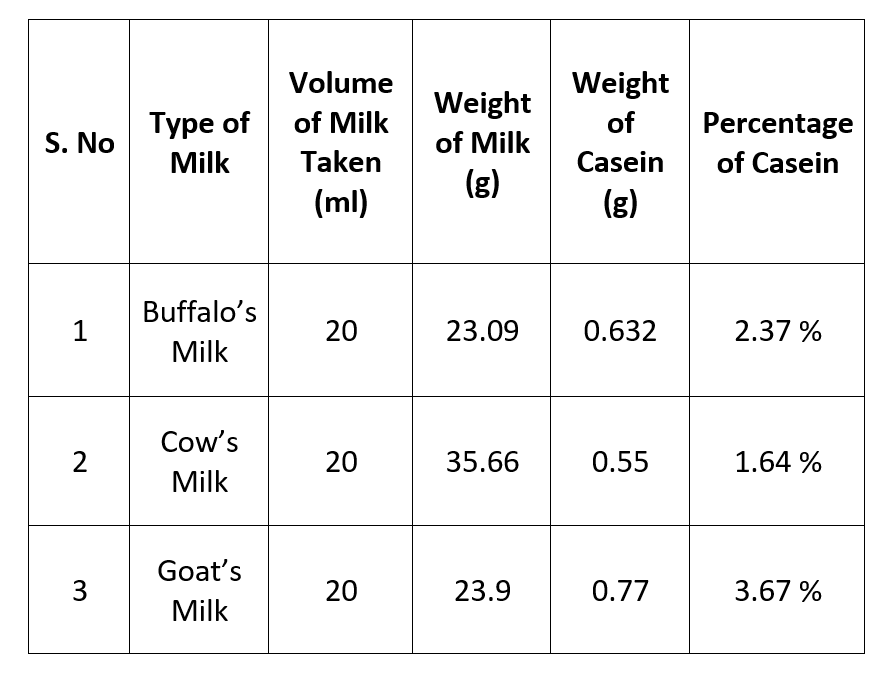
- Volume of milk taken in each case = 20ml
- Weight of milk taken = W1g
- Weight of Casein isolate = W2g
- Percentage of casein = {Weight of casein (W2)/Weight of casein (W1) } * 100
RESULT
- Different Samples of milk contains different percentage of casein.
- Highest percentage of casein is present in Goat’s milk.
APPLICATION
- In addition to being consumed in milk casein is used in the manufacture of:
Adhesives, binders, protective coatings, plastics (such as knife handles and knitting needles), fabrics, food additives, and many other products.
- It is commonly used by body builders as slow digesting whey proteins and also as an extremely high source of glutamine (post workout).
- Another reason, it is used in bodybuilding is because of its anti-catabolic effect, meaning that casein consumption inhibits protein breakdown in the body. Casein is frequently found in non-dairy substitutes to improve consistency especially when melted.
PRECAUTIONS
- Handle apparatus and chemicals carefully.
- Add ammonium sulphate solution very slowly.
- Stir milk while adding chemicals.
- Do not disturb milk after adding ammonium sulphate solution and wait some time for fat and casein to precipitate out.
- Take the amount readings carefully with digital weighing machine only.

